Hard gelatin capsules are solid dosage forms in which one or more drug substance and/or inert materials are enclosed within a small shell. They are composed largely of gelatin and consist of two parts: the body designed to contain the drug and the diluent, and the cap that is approximately half as long as the body. Most capsule products manufactured today are of the hard gelatin type. It is estimated that the utilization of hard gelatin capsules to prepare solid dosage forms exceeds that of soft gelatin capsules by about 10-fold.
Hard gelatin capsules are fabricated and supplied empty to the pharmaceutical industry by shell suppliers and are then filled in a separate operation. Manufacturing gelatin capsules involves a step by step process that requires strict quality control but before we delve into that let’s take a look at the raw materials used in the manufacture of hard gelatin capsules.
Contents
Hard gelatin capsule shell is composed largely of gelatin. Other than gelatin, it may contain materials such as plasticizer, colourants, opacifying agents, and preservatives which either enable capsule formation or improve their performance. Hard gelatin capsules also contain 12–16% water, but the water content can vary, depending on the storage conditions.
Gelatin is by far the most common and most well-known material used to produce hard capsule shells. It is a generic term for a mixture of purified protein fractions obtained from irreversible hydrolytic extraction of collagen obtained from the skin, white connective tissue, and bones of animals.
Depending on the source of the collagen and the method of extraction, two types of gelatin can be produced – type A gelatin and type B gelatin. Type A gelatin is made from pork skin via acid hydrolysis and has an isoelectric point between 7.0 and 9.0. Type B gelatin is prepared by alkaline hydrolysis of bovine bones and has an isoelectric point between 4.8 and 5.0. Because of this difference in isoelectric points, both gelatins show solubility differences at different pH values.
Traditionally capsules may be manufactured by using both types of gelatin, but combinations of pork skin and bone gelatin are often used to optimize shell characteristics because bone gelatin contributes firmness, whereas pork skin gelatin contributes plasticity and clarity.
Gelatin derived from Gelatin grade is further specified by bloom strength. This is measured in a Bloom gelometer which determines the weight in grams that is required to depress a standard plunger in a 6.67% w/w gel under standard conditions.
Gelatin is stable in air when dry but is subject to microbial decomposition when it becomes moist.
Plasticizers are added to gelatin to reduce the rigidity of the polymer and make it more pliable. Common examples of plasticizers are glycerine and polyhydric alcohol. Water is also a good plasticizer and is naturally present in the gelatin.
Most frequently, hard gelatin capsules are coloured to enhance the aesthetic properties and also to act as a means of identifying the product. Colorants used must meet the regulatory requirements of those countries where the product will be sold. Examples of commonly used capsule colourants include synthetic dyes such as azo dyes and xanthene dyes. Iron oxide pigments are also used.
Opacifiers (e.g., titanium dioxide) may be included to make clear gelatin opaque. Opaque capsules may be employed to provide protection against light or to conceal the contents.
Preservatives (often parabens esters) were formerly added to hard capsules as an in-process aid in order to prevent microbiological contamination during manufacture. Manufacturers operating their plants to Good Manufacturing Practice (GMP) guidelines no longer use them. In the finished capsules, the moisture levels, 12–16% w/ v, are such that the water activity will not support bacterial growth because the moisture is too strongly bound to the gelatin molecule.
Read Also: Hard Gelatin Capsules: Formulation and Manufacturing Considerations
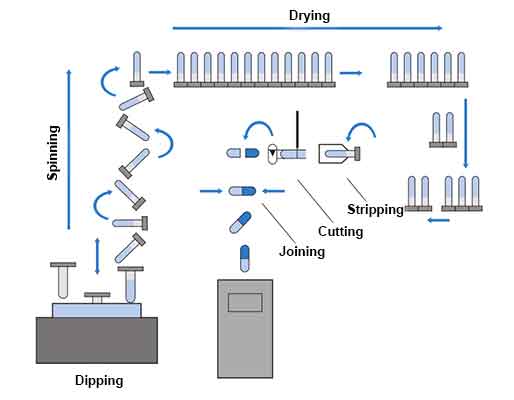
Hard gelatin capsules are manufactured using a dip-coating method and the various stages involved are as follows:
A concentrated solution of gelatin is prepared by dissolving the gelatin in demineralized water which has been heated to 60–70°C in jacketed pressure vessels. This solution contains 30 – 40% w/w of gelatin and is highly viscous, which causes bubbles as a result of air entrapment. The presence of these bubbles in the final solution would yield capsules of inconsistent weight and would also become problematic during capsule filling and upon storage. To remove the air bubbles, a vacuum is applied to the solution; the duration of this process varies with batch size.
Following the above steps, colourants and pigments are added to attain the desired final capsule appearance. At this stage, other processing aids may be added, such as sodium lauryl sulfate, to reduce surface tension. The solution viscosity is measured and adjusted as needed with hot demineralized water to achieve the target specification.
The viscosity of the gelatin solution is a critical parameter as it affects the downstream manufacturing process and plays a major role in capsule shell wall thickness. After physical, chemical, and microbiological testing, the gelatin is released for capsule production. The gelatin solution is then transferred to temperature-controlled tanks on the dipping machine where it is fed continuously into the dipping dishes.
Capsule shells are manufactured under strict climatic conditions by dipping pairs (body and cap) of standardized steel pins arranged in rows on metal bars into an aqueous gelatin solution (25 – 30% w/w) maintained at about 50 ° C in a jacketed heating pan. Because the moulds are below the gelling temperature, the gelatin begins to form a thin gelatin layer or film on the moulds.
The rows of pins are arranged so that caps are formed on one side of the machine while bodies are simultaneously formed on the opposite side of the machine.
Following adsorption of the gelatin solution on to the surface of the pins, the bar containing the pins is removed and rotated several times to evenly distribute the solution around the pins, correct gelatin distribution being critical to uniform and precise capsule wall thickness and dome strength.
Once the gelatin is evenly distributed on the mould, a blast of cool air is used to set the gelatin on the mould. At this point, the gelatin is dried, and the pins are then passed through several drying stages to achieve the target moisture content.
After the gelatin is dried, the capsule is stripped off the mould and trimmed to the proper length.
Once trimmed, the two halves (the cap and body) are joined to the pre-closed position using a pre lock mechanism. At this point, printing is done if needed before packing in cartons for shipping.
After formation, the capsule shells can be printed to improve identification. Printing can be achieved using one or two colours, containing information such as product name or code number, manufacturer’s name or logo and dosage details.
Printing reduces the risk of product confusion by the numerous handlers and users of the product including manufacturers, pharmacists, nurses, doctors, caregivers, and patients.
Click to read more on filling of hard gelatin capsules
This article titled Manufacture of Hard Gelatin Capsules focuses on the various stages involved in the manufacture of hard gelatin capsules. You can read more the manufacture of hard gelatin capsules on www.capsugel.com other related keywords: Related keyword: hard gelatin capsule manufacturing process pdf, formulation of hard gelatin capsules slideshare, hard gelatin capsules pdf, hard gelatin capsules definition, difference between hard gelatin capsule and soft gelatin capsule slideshare, raw material for hard gelatin capsules, manufacturing of soft gelatin capsule, hard gelatin capsules examples
Comments2
Superb
Thanks for the compliment.